Multiple Choice
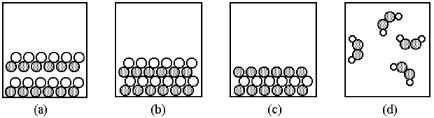
-Which of the above pictures are more likely to represent ionic compounds?
A) pictures (a) and (b)
B) pictures (a) and (d)
C) pictures (b) and (c)
D) pictures (b) and (d)
Correct Answer:

Verified
Correct Answer:
Verified
Q47: The number of electrons in the ion
Q48: Which ionic compound would be expected to
Q49: Consider the following ground state electron configuration:
Q50: Which ionic compound would be expected to
Q51: Which ion has the smallest ionic radius?<br>A)F<sup>-</sup><br>B)Cl<sup>-</sup><br>C)Br<sup>-</sup><br>D)I<sup>-</sup>
Q53: Which ion has the largest radius? Ca<sup>+2</sup>,Ca<sup>+1</sup>,Br<sup>-</sup>,K<sup>+</sup><br>A)Ca<sup>+2</sup><br>B)Ca<sup>+1</sup><br>C)Br<sup>-</sup><br>D)K<sup>+</sup>
Q54: The four spheres below represent Na<sup>+</sup>,Mg<sup>2+</sup>,F⁻,and O<sup>2-</sup>,not
Q55: Of the following,which element has the highest
Q56: In the reaction of sodium metal with
Q57: The ion that has 28 protons and