Multiple Choice
The following pictures represent alkali halide salts. 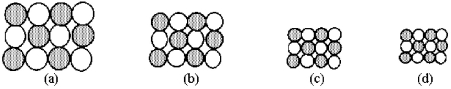
-Which salt has the lowest lattice energy?
A) picture (a)
B) picture (b)
C) picture (c)
D) picture (d)
Correct Answer:

Verified
Correct Answer:
Verified
Q53: Which ion has the largest radius? Ca<sup>+2</sup>,Ca<sup>+1</sup>,Br<sup>-</sup>,K<sup>+</sup><br>A)Ca<sup>+2</sup><br>B)Ca<sup>+1</sup><br>C)Br<sup>-</sup><br>D)K<sup>+</sup>
Q54: The four spheres below represent Na<sup>+</sup>,Mg<sup>2+</sup>,F⁻,and O<sup>2-</sup>,not
Q55: Of the following,which element has the highest
Q56: In the reaction of sodium metal with
Q57: The ion that has 28 protons and
Q59: List the elements Cs,Ca,Ne,Na,Ar in order of
Q60: An element Y,has the following ionization energies
Q61: Which of the following three sets consist
Q62: Which of the following most likely represent
Q63: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which of the