Multiple Choice
Which is the longest bond?
A) N-N
B) N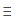 N
N
C) N=N
D) All three bond lengths should be about the same.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: Identify the set of hybrid orbitals shown
Q4: Which drawing represents the lowest energy unoccupied
Q6: A molecular model of BCl<sub>3</sub> is shown
Q7: Which drawing represents a π<sup>*</sup> antibonding molecular
Q10: Which of the following are allowed resonance
Q12: Among the compounds H<sub>3</sub>C-CH<sub>3</sub>,H<sub>2</sub>C=CH<sub>2</sub>,and HC=CH,the compound with
Q13: Which of the following should be nonplanar?
Q148: The hybrid orbital used by nitrogen to
Q162: When an electron is added to the
Q189: Which of the following is not true?<br>A)The