Multiple Choice
Consider a molecule with the following connections: 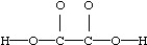
When a valid electron dot structure is written,how many double bonds will the molecule contain?
A) 0
B) 1
C) 2
D) 4
Correct Answer:

Verified
Correct Answer:
Verified
Q4: How many resonance structures are required in
Q13: Which molecular orbital resembles a d-orbital?<br>A)σ<br>B)σ<sup>*</sup><br>C)π<br>D)π<sup>*</sup>
Q38: Given that O<sub>2</sub> is paramagnetic and has
Q51: Based on formal charge considerations,the electron-dot structure
Q62: Which is the best description of the
Q129: What is the geometry around the central
Q130: Which element can accommodate more than eight
Q133: What is the bond angle in the
Q136: What are the bond angles in the
Q154: What is geometry around the nitrogen atom?<br>A)bent<br>B)tetrahedral<br>C)trigonal