Multiple Choice
Assign formal charges to each atom in the resonance form for SOCl2 given below. 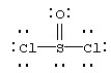
A) 0 for Cl,0 for S,and 0 for O
B) 0 for Cl,+1 for S,and -1 for O
C) -1 for Cl,+4 for S,and -2 for O
D) -1 for Cl,-2 for S,and -2 for O
Correct Answer:

Verified
Correct Answer:
Verified
Q6: Of the following elements,which has the lowest
Q36: Of the following elements,which has the lowest
Q38: How many lone pairs of electrons are
Q40: The phosphorus atom in PCl<sub>3</sub> would be
Q41: What are the bond angles in the
Q42: What is the geometry around the central
Q44: What are the bond angles in the
Q46: What is the molecular geometry of TeCl<sub>4</sub>?<br>A)seesaw<br>B)square
Q51: The VSEPR model predicts the H-O-H bond
Q79: Which molecule has the weakest bonds?<br>A)CF<sub>4</sub><br>B)CCl<sub>4</sub><br>C)CBr<sub>4</sub><br>D)CI<sub>4</sub>