Multiple Choice
The MO diagram below is appropriate for B2.Based on this diagram,B2 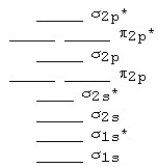
A) has a bond order of one and is diamagnetic.
B) has a bond order of one and is paramagnetic.
C) has a bond order of two and is diamagnetic.
D) has a bond order of two and is paramagnetic.
Correct Answer:

Verified
Correct Answer:
Verified
Q95: What are the bond angles in the
Q96: Compound A is a solid with a
Q97: What is the smallest bond angle in
Q97: What is the geometry around the central
Q99: Which drawing represents the molecular orbital containing
Q101: A molecular model of NO<sub>3</sub><sup>-</sup> is shown
Q103: The compound ICl contains<br>A)ionic bonds.<br>B)nonpolar covalent bonds.<br>C)polar
Q105: Elements that can accommodate more than eight
Q119: Which molecular orbital resembles a p-orbital?<br>A)σ<br>B)σ<sup>*</sup><br>C)π<br>D)π<sup>*</sup>
Q155: The following ball-and-stick molecular model is a