Multiple Choice
Use the following MO diagram for Be2,Be2+,and Be2-.Based on this diagram, 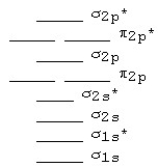
A) Be2+ is more stable than Be2,and Be2 is more stable than Be2-.
B) Be2- is more stable than Be2,and Be2 is more stable than Be2+.
C) Be2+ and Be2- are both more stable than Be2.
D) Be2 is more stable than either Be2+ or Be2-.
Correct Answer:

Verified
Correct Answer:
Verified
Q9: Predict whether removing two electrons from F<sub>2</sub>
Q35: What is the molecular geometry of SF<sub>5</sub><sup>-</sup>?<br>A)octahedral<br>B)seesaw<br>C)square
Q46: What is the molecular geometry of TeCl<sub>4</sub>?<br>A)seesaw<br>B)square
Q60: Which is paramagnetic?<br>A)N<sub>2</sub><sup>2+</sup><br>B)N<sub>2</sub><sup>2-</sup><br>C)O<sub>2</sub><sup>2+</sup><br>D)O<sub>2</sub><sup>2-</sup>
Q113: A triple bond is generally composed of<br>A)three
Q147: The carbon-carbon bond in C<sub>2</sub>H<sub>2</sub> contains _
Q205: How many lone pairs of electrons are
Q209: The molecular geometry of COCl<sub>2</sub> is _.
Q212: Using only the elements Ba,F,and P,give the
Q213: What is the hybridization of the carbon