Multiple Choice
What is the geometry around the central atom in the following molecular model of BCl3? 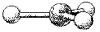
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: Ionic compounds consist of a single three-dimensional
Q61: What geometric arrangement of charge clouds is
Q64: Which type of bond produces a charge
Q96: The paramagnetism of O<sub>2</sub> is explained by<br>A)coordinate
Q174: Which molecule contains the most easily broken
Q175: Which of the following would be expected
Q176: What are the bond angles in the
Q177: What is the geometry around the central
Q178: Which of the following contains an atom
Q180: The C=O bond in COCl<sub>2</sub> can be