Multiple Choice
What is the geometry around the central atom in the following molecular model of NO3-? 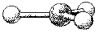
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q13: How many double and single bonds are
Q18: According to molecular orbital theory,is the highest
Q21: How many of the σ bonds in
Q36: A::A represents<br>A)a double bond.<br>B)a quadruple bond.<br>C)one lone
Q67: What is the molecular geometry of IF<sub>5</sub>?<br>A)octahedral<br>B)seesaw<br>C)square
Q68: Electrostatic potential maps use color to portray
Q70: Electrostatic potential maps use color to portray
Q71: Which of the following should be nonlinear?
Q75: What are the bond angles in the
Q76: What orbital hybridization is expected for the