Multiple Choice
What is the geometry around the central atom in the following molecular model of H2S? 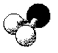
A) linear
B) bent
C) trigonal pyramidal
D) tetrahedral
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: Predict whether removing two electrons from F<sub>2</sub>
Q63: What is the molecular geometry of NCl<sub>3</sub>?<br>A)T-shaped<br>B)tetrahedral<br>C)trigonal
Q113: A triple bond is generally composed of<br>A)three
Q147: The carbon-carbon bond in C<sub>2</sub>H<sub>2</sub> contains _
Q150: The number of sp<sup>2</sup> hybrid orbitals on
Q212: Using only the elements Ba,F,and P,give the
Q213: What is the hybridization of the carbon
Q218: What are the bond angles in the
Q219: Electrostatic potential maps use color to portray
Q220: What is the hybridization of the oxygen