Multiple Choice
What is the bond angle in the following molecular model of SO2? 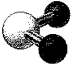
A) less than 109.5°
B) 109.5°
C) less than 120° but greater than 109.5°
D) 120°
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q15: The Lewis electron-dot structure of N<sub>2</sub> has
Q25: How many lone pairs are on the
Q28: What is the molecular geometry of ClF<sub>4</sub>
Q50: Of the following elements,which has the highest
Q52: When melting S<sub>8</sub>,_ forces must be overcome
Q93: How many lone pairs of electrons are
Q149: Which compound is most likely to exist
Q150: Which of the following contains an atom
Q158: What are the bond angles in the
Q165: The hybrid orbital used by carbon to