Multiple Choice
Shown below is a model of SiCl4 having an orientation in which one or more atoms are hidden from view.What is the indicated bond angle? 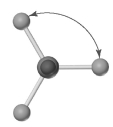
A) less than 109.5°
B) 109.5°
C) less than 120° but greater than 109.5°
D) 120°
Correct Answer:

Verified
Correct Answer:
Verified
Q63: What is the molecular geometry of NCl<sub>3</sub>?<br>A)T-shaped<br>B)tetrahedral<br>C)trigonal
Q63: Which compound is most likely to exist
Q118: What is the hybridization on the N
Q123: The following ball-and-stick molecular model is a
Q166: Which molecular orbitals for homonuclear diatomic molecules
Q181: Of XeF<sub>2 </sub>and XeF<sub>4,</sub>the one with the
Q219: Electrostatic potential maps use color to portray
Q220: What is the hybridization of the oxygen
Q222: What is the geometry around the central
Q223: What are the bond angles in the