Multiple Choice
Identify the set of hybrid orbitals shown below. 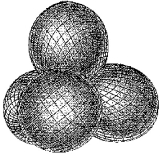
A) sp
B) sp2
C) sp3
D) None of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: Which electrostatic forces hold atoms together in
Q31: Of H<sub>2</sub>CO and CO and CO<sub>2</sub>,the compound
Q36: A::A represents<br>A)a double bond.<br>B)a quadruple bond.<br>C)one lone
Q75: What are the bond angles in the
Q76: What orbital hybridization is expected for the
Q83: What is the geometry around the central
Q84: What is the geometry around the central
Q85: What is the geometry around the central
Q90: How many lone pairs of electrons are
Q201: Which of the following is not a