Multiple Choice
A molecular model of SO42- is shown below.Based on the best Lewis electron-dot structure for SO42- and formal charge considerations,what is the predicted S-O bond order for each S-O bond? 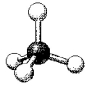
A) 1
B) 1) 33
C) 1) 5
D) 2
Correct Answer:

Verified
Correct Answer:
Verified
Q13: Which molecular orbital resembles a d-orbital?<br>A)σ<br>B)σ<sup>*</sup><br>C)π<br>D)π<sup>*</sup>
Q62: Which is the best description of the
Q76: Based on formal charges,the best Lewis electron-dot
Q133: What is the bond angle in the
Q136: What are the bond angles in the
Q137: What is the molecular geometry of AsCl<sub>3</sub>?<br>A)T-shaped<br>B)tetrahedral<br>C)trigonal
Q139: Which bond should have the highest bond
Q141: Identify the fourth-row element X that forms
Q142: What is the bond angle in the
Q159: The SO<sub>3</sub> molecule can be described as