Multiple Choice
Assume that you have a sample of gas in a cylinder with a moveable piston,as shown in diagram (1) .The initial pressure,number of moles,and temperature of the gas are noted on the diagram.Which diagram (2) -(4) most closely represents the result of doubling the number of moles of gas while keeping the pressure and temperature constant? 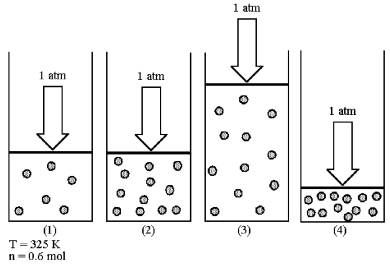
A) diagram (2)
B) diagram (3)
C) diagram (4)
Correct Answer:

Verified
Correct Answer:
Verified
Q14: "Equal volumes of different gases at the
Q48: At what temperature will sulfur hexafluoride molecules
Q104: Which statement about real gases is true?<br>A)The
Q112: The volume of 350.mL of gas at
Q120: Three bulbs,two of which contain different gases
Q124: In the laboratory,hydrogen gas is usually made
Q125: A container filled with gas is connected
Q126: In the upper atmosphere,photochemical reactions involving organic
Q127: According to the kinetic molecular theory of
Q128: When 14.0 g of zinc metal reacts