Multiple Choice
Assume that you have a sample of gas in a cylinder with a moveable piston,as shown in diagram (1) .The initial pressure,number of moles,and temperature of the gas are noted on the diagram.Which diagram (2) -(4) most closely represents the result of doubling the temperature while keeping the pressure and number of moles of gas constant? 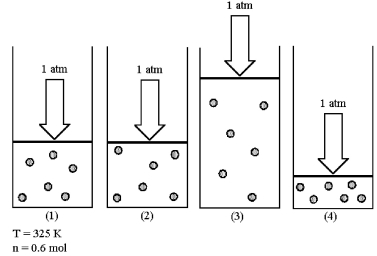
A) diagram (2)
B) diagram (3)
C) diagram (4)
Correct Answer:

Verified
Correct Answer:
Verified
Q74: Each of three identical 15.0-L gas cylinders
Q103: How many liters of SO<sub>3</sub>(g)are produced at
Q105: A glass tube has one end in
Q151: A glass tube has one end in
Q153: A gas bottle contains 0.650 mol of
Q154: If the number of moles of gas
Q155: The action of some commercial drain cleaners
Q159: What is the average speed (actually the
Q160: Each of three identical 15.0-L gas cylinders
Q162: In the diagram below,nitrogen molecules are represented