Multiple Choice
Assume that you have a sample of gas in a cylinder with a moveable piston,as shown in diagram (1) .The initial pressure,number of moles,and temperature of the gas are noted on the diagram.Which diagram (2) -(4) most closely represents the result of doubling the pressure and number of moles of gas while keeping the temperature constant? 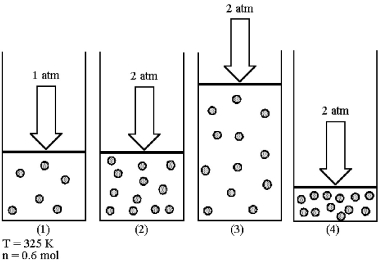
A) diagram (2)
B) diagram (3)
C) diagram (4)
Correct Answer:

Verified
Correct Answer:
Verified
Q1: The principal cause of man-made ozone depletion
Q3: A 75.0 L steel tank at 20.0°C
Q5: What is the value of the gas
Q7: In the laboratory,hydrogen gas is usually made
Q8: If the pressure in a gas container
Q22: If CO<sub>2</sub> and NH<sub>3</sub> are allowed to
Q26: Some assumptions from the kinetic molecular theory
Q53: If 1.0 mol of N<sub>2</sub> and 3.0
Q70: Suppose you needed to closely monitor small
Q146: In the diagram below,helium atoms are represented