Multiple Choice
Effusion of a 1:1 mixture of two gases,represented by unshaded and shaded spheres in the diagram below,through a small pinhole produces the result shown below.The shaded spheres have a molecular mass of 32 amu.Which gas molecules have the higher average speed and what is the molecular mass of the unshaded molecules? 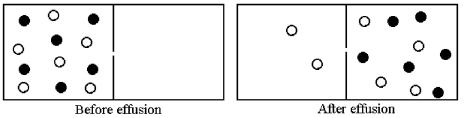
A) Unshaded molecules have higher average speed and molecular mass = 14 amu
B) Unshaded molecules have higher average speed and molecular mass = 21 amu
C) Unshaded molecules have lower average speed and molecular mass = 48 amu
D) Unshaded molecules have lower average speed and molecular mass = 72 amu
Correct Answer:

Verified
Correct Answer:
Verified
Q6: Which is the smallest quantity of pressure?<br>A)1
Q57: A gas occupies 22.4 L at STP
Q58: If NO<sub> </sub> and NH<sub>3</sub> are allowed
Q64: In a flask containing 2.00 mol of
Q65: Which of the following gases has the
Q68: Chloroform is a volatile liquid once commonly
Q124: In an open end manometer,one end of
Q129: If 1.0 gram each of Cl<sub>2</sub>,CO<sub>2</sub>,N<sub>2</sub>,and O<sub>2</sub>
Q153: Under the same pressure and temperature conditions,the
Q180: Which of the following regions of the