Multiple Choice
Effusion of a 1:1 mixture of two gases,represented by unshaded and shaded spheres in the diagram below,through a small pinhole produces the result shown below.The shaded spheres have a molecular mass of 20 amu.Which gas molecules have the higher average speed and what is the molecular mass of the unshaded molecules? 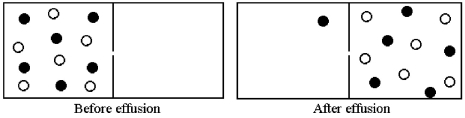
A) Unshaded molecules have higher average speed and molecular mass = 14 amu
B) Unshaded molecules have higher average speed and molecular mass = 17 amu
C) Unshaded molecules have lower average speed and molecular mass = 24 amu
D) Unshaded molecules have lower average speed and molecular mass = 29 amu
Correct Answer:

Verified
Correct Answer:
Verified
Q81: A lungful of air (500 mL)contains 4.1%
Q101: An unknown gas contains 83% C and
Q108: Which of the following gases has the
Q110: Gases do not behave ideally under conditions
Q115: According to Graham's law,the rate of effusion
Q127: According to the kinetic molecular theory of
Q128: When 14.0 g of zinc metal reacts
Q131: Each of three identical 15.0-L gas cylinders
Q133: How many grams of O<sub>2</sub> gas are
Q136: A steel bottle contains argon gas at