Multiple Choice
From the plot of vapor pressure as a function of temperature shown below,the normal boiling point for tert-butyl alcohol is approximately 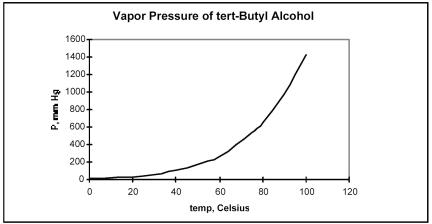
A) 0°C.
B) 40°C.
C) 85°C.
D) 100°C.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: The temperature and pressure at which all
Q17: First-order diffraction of X-rays with d =
Q42: Which covalent bond is the most polar?<br>A)N-F<br>B)C-F<br>C)Cl-F<br>D)F-F
Q56: What phase changes occur when the pressure
Q62: Rhodium has a face-centered cubic structure and
Q63: Which of the following molecules does not
Q67: Which is expected to have the largest
Q71: What is the edge length of a
Q85: When cubic unit cells stack together,how many
Q91: The normal boiling point occurs when the<br>A)intermolecular