Multiple Choice
Drawing (1) shows a system in which an equilibrium exists between dissolved and undissolved gas particles at P = 1 atm.According to Henry's law,if the pressure is increased to 2 atm and equilibrium is restored,which drawing (2) -(5) best represents the equilibrium at 2 atm? 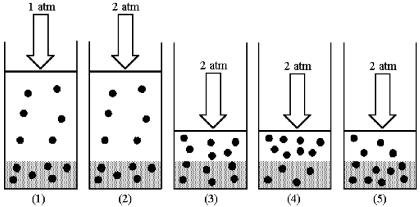
A) drawing (2)
B) drawing (3)
C) drawing (4)
D) drawing (5)
Correct Answer:

Verified
Correct Answer:
Verified
Q24: Freezing point depression,boiling point elevation,vapor pressure lowering,and
Q55: A 1.0 m aqueous CaCl<sub>2</sub> solution will
Q58: Which cation in each set would be
Q59: Stainless steel is an example of a
Q61: The number of moles of ions in
Q62: Drawing (1)shows a system in which an
Q63: At a given temperature the vapor pressures
Q65: Molarity is defined as moles of solute
Q87: What is the mole fraction of I<sub>2</sub>
Q156: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which drawing above