Multiple Choice
What is the freezing point of a solution of 7.15 g MgCl2 in 100 g of water? Kf for water is 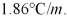
A) -0.140°C
B) - 1.40°C
C) - 2.80°C
D) - 4.18°C
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q24: Freezing point depression,boiling point elevation,vapor pressure lowering,and
Q49: How much water must be added to
Q50: Two beakers,one with pure water (light gray)and
Q55: A 1.0 m aqueous CaCl<sub>2</sub> solution will
Q58: Which cation in each set would be
Q59: Stainless steel is an example of a
Q81: Concentrations of fluoride in drinking water greater
Q87: What is the mole fraction of I<sub>2</sub>
Q135: When ethylene glycol,HOCH<sub>2</sub>CH<sub>2</sub>OH,is added to the water
Q174: When two similar liquids mix to form