Multiple Choice
What is the equilibrium equation for the following reaction?
FeS(s) + 2 H3O+ (aq) ⇌ Fe2+(aq) + H2S (aq) + 2 H2O (l)
A) Kc = 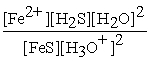
B) Kc = 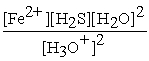
C) Kc = 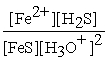
D) Kc = 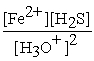
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: For the reaction shown below the equilibrium
Q23: Given the reaction: 2 HI ⇌ H<sub>2</sub>
Q99: Write the equilibrium equation for the reverse
Q102: If K<sub>c</sub> = 0.900,and K<sub>p</sub> = 538
Q104: Shown below is a concentration vs.time plot
Q106: Gaseous hydrogen bromide decomposes at elevated temperatures
Q107: Nitric oxide reacts with oxygen to form
Q111: In a reversible reaction,when the rate of
Q123: Phosphorus pentachloride decomposes to phosphorus trichloride at
Q162: What is the equilibrium constant,K<sub>c</sub>,for the reaction: