Multiple Choice
Shown below is a concentration vs.time plot for the reaction A ⇌ 2B.For this reaction the value of the equilibrium constant is 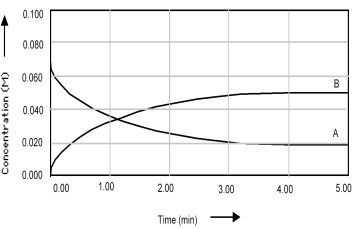
A) Kc < 1.
B) Kc = 0.
C) Kc = 1.
D) Kc > 1.
Correct Answer:

Verified
Correct Answer:
Verified
Q38: The following pictures represent mixtures of A<sub>2</sub>B<sub>4</sub>
Q40: Cyclohexane (C<sub>6</sub>H<sub>12</sub>)undergoes a molecular rearrangement in the
Q45: Which statement about the equilibrium constant is
Q46: For acid solutions of the same molarity
Q47: Find the equilibrium constant for the reaction:
Q53: The equilibrium equation is also known as
Q65: A catalyst increases the rate of a
Q97: As a rule,which of the following phases
Q156: Picture (1)represents an equilibrium mixture of solid
Q170: Picture (1)represents the equilibrium mixture for the