Multiple Choice
Shown below is a concentration vs.time plot for the reaction A ⇌ B.For this reaction the value of the equilibrium constant is 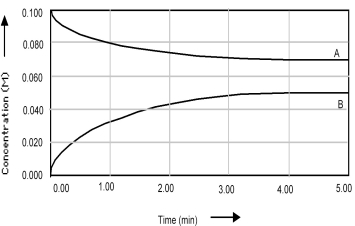
A) Kc < 1.
B) Kc = 0.
C) Kc = 1.
D) Kc > 1.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q17: According to Le Châtelier's principle,if the volume
Q29: Given the reaction at a certain temperature:
Q46: For acid solutions of the same molarity
Q47: Find the equilibrium constant for the reaction:
Q52: The decomposition of nitrosyl bromide is exothermic:
Q53: The overall reaction for photosynthesis can be
Q55: Cyclohexane,C<sub>6</sub>H<sub>12</sub>,undergoes a molecular rearrangement in the presence
Q144: The solubility of 1:1 salts is measured
Q156: Picture (1)represents an equilibrium mixture of solid
Q160: For the reaction 2 A + B<sub>2</sub>