Multiple Choice
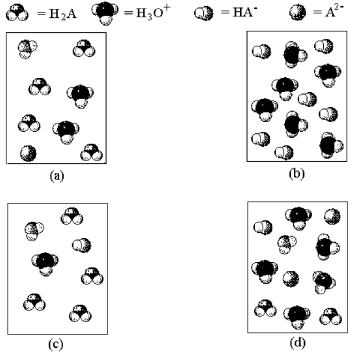
-Which of the above pictures represents a solution of a diprotic acid H2A for which Ka1 = and Ka2 is exceptionally small.(Water molecules have been omitted for clarity. )
A) picture (a)
B) picture (b)
C) picture (c)
D) picture (d)
Correct Answer:

Verified
Correct Answer:
Verified
Q14: What is the hydronium ion concentration of
Q16: Calculate the pH of a 0.080 M
Q19: What is the approximate pH of a
Q20: Which one of the following salts,when dissolved
Q22: Identify the set of Lewis acids.<br>A)BH<sub>3</sub>,BF<sub>3</sub>,Cu<sup>2+</sup>,CO<sub>2</sub><br>B)Cl<sup>-</sup>,OH<sup>-</sup>,NH<sub>3</sub>,H<sub>2</sub>O<br>C)H<sub>3</sub>PO<sub>4</sub>,H<sub>2</sub>PO<sub>4</sub><sup>-</sup>,HPO<sub>4</sub><sup>2-</sup>,PO<sub>4</sub><sup>3-</sup><br>D)CH<sub>3</sub><sup>-</sup>,NH<sub>2</sub><sup>-</sup>,OH<sup>-</sup>,F<sup>-</sup>
Q134: Bromothymol blue indicator changes color from yellow
Q170: An acidic solution at 25°C has<br>A)[H<sub>3</sub>O<sup>+</sup>] >
Q175: The value of K<sub>a</sub> for a 0.250
Q177: At 50°C the value of K<sub>w</sub> is
Q196: In the following reaction the unshaded spheres