Multiple Choice
What is the equation relating the equilibrium constant Kn for the neutralization of a weak acid with a weak base to the Ka of the acid,the Kb of the base and Kw?
A) Kn = KaKbKw
B) Kn = 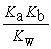
C) Kn = 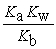
D) Kn = 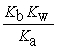
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Calculate the molar solubility of thallium(I)chloride in
Q31: The following pictures represent solutions of CaCO<sub>3</sub>,which
Q85: What is the pH of a buffered
Q100: Which is the best acid to use
Q104: The dissociation equilibrium constants for the protonated
Q105: Which is a net ionic equation for
Q113: A buffer prepared by mixing 50.00 mL
Q121: The following plot shows a titration curve
Q131: Sodium hypochlorite,NaOCl,is the active ingredient in household
Q161: The following plot shows two titration curves,each