Multiple Choice
Shown below is a galvanic cell with anode compartment b containing anode a and cathode compartment d containing cathode c.Electrons flow through wire f,ions flow through salt bridge e,and the cell potential is read using voltmeter g.
This galvanic cell uses the reaction: Cu(s) + 2 Ag+(aq) 2 Ag(s) + Cu2+(aq) . 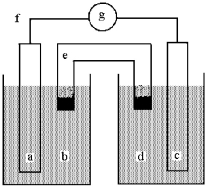
-The initial concentrations of Ag+(aq) and Cu2+(aq) are both 1.0 M.What will happen to the cell voltage if 1.0 M Cu(NO3) 2 is added to the compartment containing the 1.0 M Cu2+(aq) ? The cell voltage will
A) decrease.
B) increase.
C) remain the same.
D) can't tell from the information given
Correct Answer:

Verified
Correct Answer:
Verified
Q36: Shown below are the reactions occurring in
Q56: For a particular battery based on one
Q69: A galvanic cell consists of one half-cell
Q81: The initial concentrations of Ag<sup>+</sup>(aq)and Cu<sup>2+</sup>(aq)are both
Q102: For an electrolytic cell<br>A)E is negative and
Q104: Calculate the value of the reaction quotient,Q,for
Q110: For a galvanic cell that uses the
Q202: Aluminum requires relatively little protection from corrosion
Q210: Which of the following statements concerning the
Q212: The galvanic cell represented by the shorthand