Multiple Choice
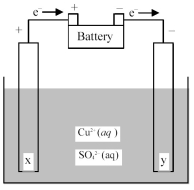
-Is the cell shown above a galvanic or an electrolytic cell? What is the direction of ion flow?
A) Electrolytic cell;Cu2+ ions flow toward electrode x and SO42- ions flow toward electrode y.
B) Electrolytic cell;Cu2+ ions flow toward electrode y and SO42- ions flow toward electrode x.
C) Galvanic cell;Cu2+ ions flow toward electrode x and SO42- ions flow toward electrode y.
D) Galvanic cell;Cu2+ ions flow toward electrode y and SO42- ions flow toward electrode x.
Correct Answer:

Verified
Correct Answer:
Verified
Q6: How long must a constant current of
Q62: The standard potential for the following galvanic
Q65: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4939/.jpg" alt=" -Use Table 17.1
Q67: A salt bridge is used to<br>A)provide reactants
Q69: Given that Cl<sub>2</sub>(g)+ 2 e<sup>-</sup> ?
Q72: Consider the galvanic cell,Pb(s)| Pb2+(aq)|| Cu<sup>2+</sup>(aq)| Cu(s).Which
Q73: Consider the galvanic cell,Zn(s)∣ Zn<sup>2+</sup>(aq)∣∣ Pb<sup>2+</sup>(aq)∣ Pb(s).Which
Q115: Calculate the cell potential at 25°C for
Q117: What is the balanced chemical equation for
Q178: In a galvanic cell,the half-reaction MnO<sub>4</sub><sup>-</sup>(aq)+ 8