Multiple Choice
What is the total volume of the mixture of hydrogen gas and oxygen gas can be obtained from the electrolysis of 90.0 grams of water at 25.0°C and 1.00 atm pressure according to the chemical equation shown below?
2 H2O(l) 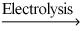 2 H2(g) + O2(g)
2 H2(g) + O2(g)
A) 61.1 L
B) 122 L
C) 183 L
D) 366 L
Correct Answer:

Verified
Correct Answer:
Verified
Q89: What is the total volume of hydrogen
Q91: What is the total volume of the
Q93: What is the total volume of hydrogen
Q94: Which is not true about elemental oxygen?<br>A)Oxygen
Q95: How many liters of hydrogen gas can
Q96: Which individual is generally given credit for
Q189: In the following pictures representing binary hydrides
Q190: There are three isotopes of oxygen <sup>16</sup>O,<sup>17</sup>O
Q258: What is the Lewis electron dot structure
Q264: Commercially oxygen is usually obtained by<br>A)decomposition of