Multiple Choice
In the following picture of an oxide,darkly-shaded spheres represent oxygen atoms or ions and lightly-shaded spheres represent atoms or ions of a third-row element in its highest oxidation state.Tell whether the oxide is covalent or ionic and whether the oxide is acidic or basic. 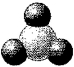
A) covalent and acidic
B) covalent and basic
C) ionic and acidic
D) ionic and basic
Correct Answer:

Verified
Correct Answer:
Verified
Q78: All of the following are reactions commonly
Q79: What is the bond length trend for
Q80: What are the three major chemicals that
Q81: Indicate the coefficient in front of H<sub>2</sub>O<sub>2</sub>
Q83: Consider the hypothetical isotope of hydrogen,quadrium,Q =
Q84: When 5.60 grams of anhydrous copper(II)sulfate is
Q85: When all water is removed from a
Q103: Using the three different isotopes of oxygen
Q190: There are three isotopes of oxygen <sup>16</sup>O,<sup>17</sup>O
Q256: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which element shown