Multiple Choice
Look at the location of elements A,B,C,and D in the following periodic table.Consider the oxides that form when the elements A-D are in their highest oxidation states. 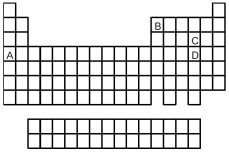
-Which oxide is the most covalent?
A) A
B) B
C) C
D) D
Correct Answer:

Verified
Correct Answer:
Verified
Q14: How many liters of hydrogen gas are
Q15: How many liters of hydrogen gas are
Q16: Which is classified as an amphoteric binary
Q17: Which isotope of diatomic hydrogen should have
Q18: How many grams of KCl(s)are produced from
Q20: Superoxide ion,O<sub>2</sub><sup>-</sup>,peroxide ion,O<sub>2</sub><sup>2-</sup>,and oxide ion,O<sup>2-</sup>,contain _,_,and _
Q21: Look at the location of elements A,B,C,and
Q22: When KO<sub>2</sub> is dissolved in water the
Q23: The dissociation of D<sub>2</sub>O is shown below.<br>D<sub>2</sub>O
Q25: How many mL of O<sub>2</sub> gas at