Multiple Choice
Look at the location of elements A,B,C,and D in the following periodic table.Consider the oxides that form when the elements A-D are in their highest oxidation states. 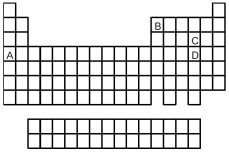
-Which oxide is the most acidic?
A) A
B) B
C) C
D) D
Correct Answer:

Verified
Correct Answer:
Verified
Q22: When KO<sub>2</sub> is dissolved in water the
Q23: The dissociation of D<sub>2</sub>O is shown below.<br>D<sub>2</sub>O
Q25: How many mL of O<sub>2</sub> gas at
Q25: How many mL of O<sub>2</sub> gas at
Q28: Which one of the following binary oxides
Q29: An example of an isotope effect is
Q30: A binary compound that forms when potassium
Q31: The largest single industrial use of hydrogen
Q32: The oxygen-oxygen bond order in ozone,O<sub>3</sub>,is _.The
Q203: The following pictures represent binary hydrides,AH<sub>x</sub>,where A