Multiple Choice
Look at the location of elements A,B,C,and D in the following periodic table.Consider the oxides that form when the elements A-D are in their highest oxidation states. 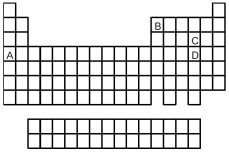
-Which oxide is a solid with an infinitely extended three-dimensional crystal structure?
A) A
B) B
C) C
D) D
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q27: What is the oxidation number of oxygen
Q95: How many liters of hydrogen gas can
Q96: Which individual is generally given credit for
Q98: The oxide of which element,identified by letters
Q99: How many grams of zinc metal are
Q101: Indicate the order of reactivity of the
Q102: What is the anhydrous form of nitric
Q103: How many grams of KCl(s)are produced from
Q104: Anhydrous magnesium sulfate can be used as
Q258: What is the Lewis electron dot structure