Multiple Choice
The following molecular orbital energy level diagram shows the energies and occupancies of the MOs derived from the atomic 2p orbitals for an oxygen-containing binary compound of potassium.This compound is a 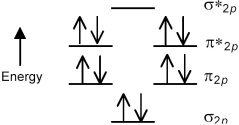
A) peroxide that is attracted by magnetic fields.
B) peroxide that is repelled by magnetic fields.
C) superoxide that is attracted by magnetic fields.
D) superoxide that is repelled by magnetic fields.
Correct Answer:

Verified
Correct Answer:
Verified
Q16: Which of the following is a peroxide?<br>A)Li<sub>2</sub>O<br>B)K<sub>2</sub>O<sub>2</sub><br>C)CaO<br>D)CsO<sub>2</sub>
Q55: Sodium hydride,NaH,reacts with water as shown in
Q57: Look at the location of elements A,B,C,and
Q58: In which set are all compounds considered
Q61: When 1.800 grams of anhydrous magnesium chloride
Q62: An element that forms a covalent hydride
Q63: In the reaction of ozone shown below,which
Q64: Covalent hydrides of the type MH<sub>3</sub> are
Q201: In the following pictures representing binary hydrides
Q205: The following pictures represent binary hydrides,AH<sub>x</sub>,where A