Multiple Choice
How many liters of oxygen gas can be produced at STP from the decomposition of 0.250 L of 3.00 M H2O2 in the reaction according to the chemical equation shown below? 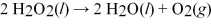
A) 8) 41 L
B) 11.2 L
C) 16.8 L
D) 33.6 L
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: How many liters of O<sub>2</sub> gas at
Q8: A 7.63-gram sample of hydrated magnesium sulfate
Q9: How many liters of hydrogen gas can
Q10: If the molar mass of monoatomic deuterium
Q13: In the preparation of oxygen by the
Q14: How many liters of hydrogen gas are
Q15: How many liters of hydrogen gas are
Q16: Which is classified as an amphoteric binary
Q17: Which isotope of diatomic hydrogen should have
Q167: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Using the above