Short Answer
The preparation of oxygen by the thermal decomposition of potassium chlorate is shown below.
2 KClO3(s) 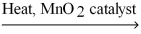
2 KCl(s)+ 3 O2(g)
In this reaction the atom that is reduced is ________,which undergoes an oxidation number change from ________ to ________.
Correct Answer:

Verified
Correct Answer:
Verified
Q27: What is the oxidation number of oxygen
Q103: How many grams of KCl(s)are produced from
Q104: Anhydrous magnesium sulfate can be used as
Q106: When 3.600 grams of anhydrous magnesium chloride
Q107: Differences in the physical properties of H<sub>2</sub>O
Q109: The strongest homonuclear single bond is<br>A)H-H<br>B)C-C<br>C)N-N<br>D)O-O
Q110: A binary compound that forms when potassium
Q111: Binary hydrides can be classified as covalent,ionic,or
Q112: A reaction occurs when 0.2105 g of
Q113: When 0.500 grams of CuSO<sub>4</sub> ∙ 5H<sub>2</sub>O