Multiple Choice
The first transition series metals are shown below. 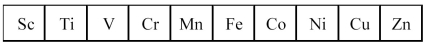
-Which has the lowest melting point?
A) Sc
B) V
C) Mn
D) Zn
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q173: The second transition series metals are shown
Q174: [Co(NH<sub>3</sub>)<sub>5</sub>NCS]<sup>2+</sup> and [Co(NH<sub>3</sub>)<sub>5</sub>SCN]<sup>2+</sup> are examples of _
Q175: What two transition elements have the highest
Q176: How many d electrons are there in
Q177: What is the oxidation state of the
Q179: In the two half-reactions shown below,which chromium
Q180: What is the strongest oxidizing agent of
Q181: The first transition series metals are shown
Q182: Which ion would you expect to have
Q183: Which of the following elements is an