Multiple Choice
The first transition series metals are shown below. 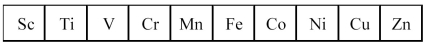
-Which has the greatest density?
A) Sc
B) V
C) Cu
D) Zn
Correct Answer:

Verified
Correct Answer:
Verified
Q39: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Using the above
Q40: What is the characteristic outer electron configuration
Q41: The oxidation state of chromium in Cr<sub>2</sub>O<sub>7</sub><sup>2-</sup>
Q42: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which
Q43: Which complex is optically active?<br>A)[CoCl<sub>4</sub>en]<sup>2-</sup><br>B)trans-[CrCl<sub>2</sub>(en)<sub>2</sub>]<sup>+</sup><br>C)cis-[CrCl<sub>2</sub>(en)<sub>2</sub>]<sup>+</sup><br>D)[PtCl<sub>2</sub>(NH<sub>3</sub>)<sub>2</sub>]
Q45: Polydentate ligands are known as _ agents.
Q46: Which of the following ions should be
Q47: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which is the
Q48: What is the ground-state electron configuration for
Q49: Which chromium species exists only under acidic