Multiple Choice
The second transition series metals are shown below. 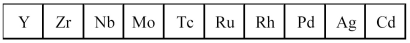
-Which has the lowest density?
A) Y
B) Mo
C) Rh
D) Cd
Correct Answer:

Verified
Correct Answer:
Verified
Q52: Which one of the following complexes is
Q53: Which one of the following compounds is
Q54: Which is a third transition series element?<br>A)B<br>B)Mg<br>C)Y<br>D)Ta
Q55: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Using the above
Q56: What is the highest possible oxidation state
Q58: The number of unpaired electrons in tetrahedral
Q59: Using the following reduction potentials for copper
Q60: The first transition series metals are shown
Q61: Based on the variation in Z<sub>eff</sub>,which M<sup>2+</sup>
Q62: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which element indicated