Multiple Choice
What is the balanced chemical equation that represents the following reaction? 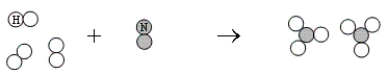
A) 6H + 2N → 2NH3
B) 6H + 2N → 2HN3
C) 2N + 2H3 → 2H3N
D) 6H + 2N → 2N3H
E) 3H2 + N2 → 2NH3
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q34: The symbol for tin is<br>A)T.<br>B)Tn.<br>C)Si.<br>D)Ti.<br>E)Sn.
Q35: Suppose atom 1 has the same number
Q36: The correct name for FeO is<br>A)iron(I)oxide.<br>B)iron oxide.<br>C)iron
Q37: What is the symbol of the nuclide
Q38: Cathode rays are<br>A)anions.<br>B)protons.<br>C)cations.<br>D)positrons.<br>E)electrons.
Q40: What is the subscript of potassium in
Q41: Which one of the following lists gives
Q42: Lithium has two naturally occurring isotopes,<sup>6</sup>Li and
Q43: The formulas of the carbonate ion,the ammonium
Q44: If the Thomson model of the atom