Monodisperse Polyacrylonitrile Contains Molecules with the General Formula -(CH2CHCN)n-,Where N
Multiple Choice
Monodisperse polyacrylonitrile contains molecules with the general formula -(CH2CHCN) n-,where n is typically greater than 10,000.Given that a sample of monodisperse polyacrylonitrile weighs 665.1 g and contains 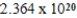 molecules of -(CH2CHCN) n-,what is the molar mass of the polymer?
molecules of -(CH2CHCN) n-,what is the molar mass of the polymer?
A) 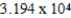 g/mol
g/mol
B) 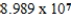 g/mol
g/mol
C) 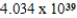 g/mol
g/mol
D) 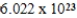 g/mol
g/mol
E) 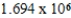 g/mol
g/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q42: A _ is used to determine the
Q43: An organic compound has a molecular mass
Q44: Styrene's empirical formula is CH.What mass of
Q45: Elemental sulfur can be converted to sulfur
Q46: _ is the calculation of the quantities
Q48: A sample containing only carbon,hydrogen,phosphorus,and oxygen is
Q49: How many moles of iron atoms are
Q50: NaHCO<sub>3</sub> is the active ingredient in baking
Q51: The total number of oxygen atoms in
Q52: A 3.903 g sample of a hydrocarbon