Multiple Choice
What is the mass of carbon in grams found in one molecule of the compound C7H8O4?
A) 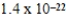 g
g
B) 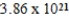 g
g
C) 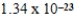 g
g
D) 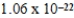 g
g
E) 156.000000 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q99: Which of the following compounds has the
Q100: How many molecules are there in 104
Q101: The limiting reactant is the reactant<br>A)that has
Q102: A compound has a molar mass of
Q103: Consider an initial mixture of CH<sub>4</sub> and
Q105: Which of the following contains the greatest
Q106: A 4.215 g sample of a compound
Q107: How many atoms of carbon are there
Q108: What is the molecular mass of the
Q109: What is the mass in grams of