Multiple Choice
All of the following reactions are described as decomposition reactions except
A) CuSO4 • 5H2O(s) 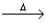 CuSO4(s) + 5H2O(g) .
CuSO4(s) + 5H2O(g) .
B) 2PbO2(s) 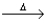 2PbO(s) + O2(g) .
2PbO(s) + O2(g) .
C) Fe(CO) 5(l) 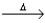 Fe(s) + 5CO(g) .
Fe(s) + 5CO(g) .
D) H2SO3(aq) 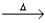 SO2(g) + H2O(l) .
SO2(g) + H2O(l) .
E) NO2(g) + H2O(l) 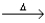 NO(g) + 2HNO3(aq) .
NO(g) + 2HNO3(aq) .
Correct Answer:

Verified
Correct Answer:
Verified
Q83: Which of the following conversions is not
Q84: What minimum mass of iron (II)nitrate must
Q85: Which of the following aqueous solutions would
Q86: All the following are oxidation-reduction reactions except<br>A)H<sub>2</sub>(g)+
Q87: In a volumetric analysis experiment,a solution of
Q89: Identify the spectator ion(s)in the following reaction.<br>Cu(OH)<sub>2</sub>(s)+
Q90: A precipitate will form when a freshly
Q91: In order to determine the amount of
Q92: All the following are strong acids in
Q93: What is the oxidation number of Ba