Multiple Choice
Which of the following is a correct statement of Charles's law, 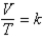 ?
?
A) The volume of a gas varies proportionally with the pressure.
B) The volume of a gas sample varies directly with the absolute temperature.
C) All gas samples of the same volume at STP contain the same number of atoms.
D) The pressure of a gas sample varies inversely with the volume.
E) All gas samples of the same volume at STP contain the same number of molecules.
Correct Answer:

Verified
Correct Answer:
Verified
Q91: A sample of oxygen is collected over
Q92: How many moles of gas are in
Q93: A 22.4 L high pressure reaction vessel
Q94: Which of the following is included as
Q95: A gas occupies a volume of 2.75
Q97: The molar mass of an unknown gas
Q98: Calcium nitrate will react with ammonium chloride
Q99: At STP,as the molar mass of the
Q100: In which of the following reactions will
Q101: The following equation represents the partial combustion