Multiple Choice
Combustion of 3.65 g of liquid benzene (C6H6) causes a temperature rise of 21.1°C in a constant-pressure calorimeter that has a heat capacity of 7.23 kJ/°C.What is ΔH for the following reaction?
C6H6(l) + 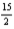 O2(g) → 6CO2(g) + 3H2O(l)
O2(g) → 6CO2(g) + 3H2O(l)
A) 150 kJ/mol
B) 41.8 kJ/mol
C) -41.8 kJ/mol
D) -3.27× 103 kJ/mol
E) 152 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q4: The reaction of iron with hydrochloric acid
Q5: Calculate ΔU of a gas for a
Q6: If q = -96 kJ for a
Q7: If q = 60 kJ and w
Q8: Which of the following processes will result
Q10: In a bomb calorimeter,reactions are carried out<br>A)at
Q11: When 50.0 mL of 1.27 M of
Q12: From the following information,determine ΔH°<sub>f</sub> of malonic
Q13: When 0.0500 mol of HCl(aq)is reacted with
Q14: The units for heat capacity are<br>A)J/g.<br>B)(J ∙