Multiple Choice
Given the following thermochemical data at 25°C and 1 atm pressure, 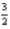 O2(g) + 2B(s) → B2O3(s) ; ΔH° = -1264 kJ
O2(g) + 2B(s) → B2O3(s) ; ΔH° = -1264 kJ
O3(g) + 2B(s) → B2O3(s) ; ΔH° = -1406 kJ
Determine ΔH° for the following reaction at 25°C and 1 atm pressure.
3O2(g) → 2O3(g)
A) -980 kJ/mol
B) +284 kJ/mol
C) +980 kJ/mol
D) -2670 kJ/mol
E) -284 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q107: How much heat is released at constant
Q108: Which of the following statements is true
Q109: The energy associated with an object held
Q110: A 3.540-g sample of an unknown metal
Q111: What is the change in enthalpy at
Q113: When 56.8 g of lead reacts with
Q114: In a certain experiment,0.7000 mol of hydrogen
Q115: Which of the following has a standard
Q116: What is ΔH° of the following reaction?<br>CO<sub>2</sub>(g)+
Q117: Given that<br>O(g)+ e<sup>-</sup> → O<sup>-</sup>(g); ΔH =