Multiple Choice
The standard enthalpy change for which of the following processes corresponds to the standard enthalpy of formation of solid lithium chloride?
A) Li(s) + 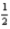 Cl2(s) → LiCl(s)
Cl2(s) → LiCl(s)
B) Li(g) + 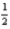 Cl2(g) → LiCl(g)
Cl2(g) → LiCl(g)
C) Li(g) + Cl(g) → LiCl(s)
D) Li(s) + 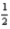 Cl2(g) → LiCl(s)
Cl2(g) → LiCl(s)
E) Li(s) + 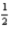 Cl2(s) → LiCl(g)
Cl2(s) → LiCl(g)
Correct Answer:

Verified
Correct Answer:
Verified
Q30: What is the change in internal energy
Q31: How much heat is liberated at constant
Q32: It is relatively easy to change the
Q33: When 9.42 g of methane (CH<sub>4</sub>)is burned
Q34: A 1.67-g sample of solid silver reacted
Q36: When 13.8 mL of 0.870 M lead(II)nitrate
Q37: Which of the following processes will result
Q38: Which of the following statements about enthalpy
Q39: What is the change in enthalpy at
Q40: Which of the following has a standard