Multiple Choice
Which of the following reactions corresponds to the thermochemical equation for the standard enthalpy of formation of N,N-diethyl-m-toluamide,C12H17NO(l) ,the active ingredient in some insect repellents?
A) 12C(l) + 17H(l) + N(l) + O(l) → C12H17NO(l)
B) 12C(g) + 17H(g) + N(g) + O(g) → C12H17NO(g)
C) 12C(s) + 17H(g) + N(g) + O(g) → C12H17NO(l)
D) 12C(s) + 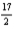 H2(g) +
H2(g) + 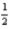 N2(g) +
N2(g) + 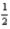 O2(g) → C12H17NO(l)
O2(g) → C12H17NO(l)
E) 12C(g) + 17H(g) + N(g) + O(g) → C12H17NO(l)
Correct Answer:

Verified
Correct Answer:
Verified
Q14: The units for heat capacity are<br>A)J/g.<br>B)(J ∙
Q15: The skydiver plunging to earth possesses what
Q16: A 58.7-g sample of nickel (s =
Q17: How much heat must be applied to
Q18: Under conditions of constant pressure,for which of
Q20: The reaction of iron with hydrochloric acid
Q21: Given the following data,calculate the standard enthalpy
Q22: Using the following data,calculate the standard enthalpy
Q23: At constant pressure and 25°C,what is ΔH°
Q24: Determine the kinetic energy of an 88.0