Multiple Choice
Which of the following sets of quantum numbers (n,l,ml, ms) refers to a 3d orbital?
A) 2 0 0 - 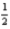
B) 5 4 1 - 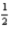
C) 4 2 -2 + 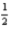
D) 4 3 1 - 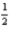
E) 3 2 1 - 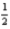
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q27: Which of the following subshells does not
Q28: How many orbitals have the set of
Q29: In Bohr's atomic theory,when an electron moves
Q30: What is the value of the spin
Q31: What is the wavelength of a 142-g
Q33: The frequency of a radio emission is
Q34: What is the wavelength of light emitted
Q35: Whose postulates account for the line spectrum
Q36: The contribution for which de Broglie is
Q37: What is the wavelength of a photon